| |
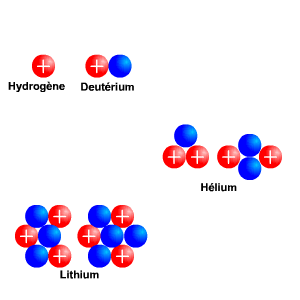
Atome Isotope. Isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, "Z") but a different number of neutrons, meaning that their mass number. The atoms of different isotopes are atoms of the same chemical element.
Learn about and revise the structure of atoms, atoms and isotopes and ions with GCSE Bitesize Physics.
Isotope ● ● ● ● Isotope sind Atome mit gleichen chemischen Eigenschaften, aber verschiedenen physikalischen Eigenschaften.
Nous décrirons aussi ce qu'est un isotope. Isotopes are atom families that have the same number of protons, but different The atomic number is roughly equivalent to an element's numeric place in the periodic table. In other words, the atoms of an element can have two or more different structures, which have the same atomic number.